How To Clean Wound Contaminated With Feces
Abstruse
Immunocompromised patients are predisposed to chronically infected wounds. Specially ulcers in the dorsal region oft experience secondary polymicrobial infections. All the same, current wound infection models mostly utilise unmarried-strain leaner. To mimic clinically occurring infections caused by fecal contamination in immunocompromised/immobile patients, which differ significantly from unmarried-strain infections, the present written report aimed at the establishment of a new mouse model using infection past fecal leaner. Dorsal round excision wounds in immunosuppressed mice were infected with fecal slurry solution in several dilutions up to i:8,000. Touch of immunosuppressor, bacterial load and timing on evolution of wound infections was investigated. Wounds were analyzed by scoring, 3D imaging and swab analyses. Autofluorescence imaging was non successful. Dose-finding of cyclophosphamide-induced immunosuppression was necessary for establishment of bacterial wound infections. Infection with fecal slurry diluted ane:166 to 1:400 induced significantly delayed wound healing (p < 0.05) without systemic reactions. Swab analyses post-infection matched the initial polymicrobial suspension. The customized wound score confirmed significant differences betwixt the groups (p < 0.05). Hither we report the establishment of a unproblematic, new mouse model for clinically occurring wound infections by fecal bacteria and the evaluation of appropriate wound assay methods. In the future, this model will provide a suitable tool for the investigation of complex microbiological interactions and evaluation of new therapeutic approaches.
Introduction
Each year, 305 meg people endure from acute, traumatic or burn wounds, globallyane. The European community spends 2–4% of the total health expenditure on wound treatment2. Wounds can arise from injuries, surgeries and as a consequence of extrinsic factors and underlying comorbidities (e.g. vascular diseases or diabetes). Hence, the general classifications differentiate between astute (due east.g. burns or surgical wounds) and chronic wounds, (e.g. vascular, diabetic or force per unit area ulcers). In salubrious individuals, the acute wounds by and large heal without complications with basic supportive care and minimal infection risk. However, comorbidities and/or risk factors such as vascular diseases, diabetes, a weak immune system, bacterial colonization (especially with pathogens of high intrinsic virulence/resistance) may often pb to the evolution of a chronically infected wounds. Peel and soft tissue infections (SSTIs) represent the most mutual infections in humans3. The associated impairment of wound healing incurs fiscal and logistic burden to the wellness intendance system and lowers the quality of life in affected patients4.
In the clinical setting, secondary infections of chronically non-healing wounds further aggravate their circuitous pathology and delay the healing processes. Immunocompromised patients are prone to develop chronically infected wounds5, and especially patients with pressure ulcers often experience secondary polymicrobial infections. Ulcer wounds typically occur in the coccyx region and due to their proximity to the sphincter and increased fecal incontinence in these, by bulk elderly patients, they are decumbent to contamination with intestinal bacteriavi. In this regard, so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp., eastward.g. Escherichia coli) are of particular importance, also, due to their frequent occurrence in nosocomial infections7,eight.
Wound infection models have been established in several species, with rodents as the most frequently used experimental platform9,ten. Well-nigh oft, wounds in these rodent models were induced by skin abrasion11,12,thirteen,14,15,xvi or burn injury17,18,19,xx,21. To restate larger wounds equally caused by ulcers and/or traumatic injuries, excision wounds are a more suitable model. Hamblin et al.22 used such a model to quantify bacterial single-strand wound infections past bioluminescence imaging23,24,25.
Infections with single, isolated pathogens have been used in the majority of pre-clinical studies, despite the fact that the majority of clinically encountered acute (and too chronic) wound infections are polymicrobial and characteristic mixed aerobic and anaerobic populations26. Preclinical models representing polymicrobial infections are sparse27,28,29, particularly using leaner derived from the aforementioned animal species. To fill up this gap, here we adult an in vivo model of feces-contaminated wound infection in immunosuppressed mice. Immunosuppression was induced by application of cyclophosphamide, which leads to neutropenia. The model comprises the infection of a dorsal total-thickness excision wound past a topical application of a fecal slurry.
Methods
Animals
12 weeks one-time female BALB/c mice (total north = 85) from Janvier Labs with an average weight of 20–25 1000 were used for the experiments. Mice were housed in groups of 4 animals per Type-III cage on a 12 h light–nighttime diurnal cycle with room temperature between 21 and 23 °C. Standard rodent nutrition (Abbedd Lab & Vet Service, Vienna, Republic of austria) and h2o were provided ad libitum throughout the experiments. Cages were enriched with carton houses, wooden boards, minor blocks for gnawing equally well as wood wool for nesting (Abbedd Lab & Vet Service, Vienna, Austria) to facilitate natural beliefs prior to and throughout the experiments. Mice were acclimated for x days before the experiments.
Cecal slurry (CS) preparation
Cecal slurry was prepared according to a modified procedure, which was previously described30. Briefly, 20 female person, 10-weeks sometime BALB/c mice were sacrificed and their whole ceca dissected. Full cecal content was collected in sterile petri dishes using sterile forceps and spatula and mixed with double distilled h2o (ddH2O) at a ratio of 0.five ml ddH2O per 0.one g of cecal content. The pause was strained through both an 800 µm and sequentially a 100 µm sieve (HAVER Exam Sieve, VWR International, Radnor, Pennsylvania). The book of the filtrate was determined and mixed with an equal amount of 30% glycerol solution in phosphate buffered saline (PBS). CS aliquots were stored at − 80 °C until use.
For infection procedure, an aliquot was thawn and centrifuged (xvi.000 g, iii min, room temperature, Biofuge Pico, Heraeus, Hanau, Deutschland). The supernatant was discarded, and the pellet was resuspended in 30 µl of 0.ix% sterile saline. This break is after indicated as stock solution. The bacteria concentration of the CS stock solution was quantified using the Leaner Counting Kit, as described below, and showed a concentration of 1 × 109/ml. All dilutions were prepared from this stock by mixture with sterile 0.9% sterile saline.
Wound model
Surgery
To induce temporary neutropenia, mice were pretreated with intraperitoneal cyclophosphamide (CPA) injections of 150 mg/kg on twenty-four hour period 4 and 100 mg/kg on mean solar day 1 before surgical procedure.31 Under inhalation anesthesia the skin was depilated, disinfected (Isozid, Gebro Pharma, Vienna) and a 1 cm circular total-thickness excision wound was cut on the dorsal median line using surgical scissors and forceps.
Polymicrobial wound infection
A interruption of 30 µl of CS was used to inoculate the wounds in the infection grouping. Control animals were treated with 30 µl of 0.9% sterile saline and their wounds were additionally disinfected during each dressing modify using Octenisept (Schülke & Mayr GmbH, Norderstedt, Frg).
Wound dressing
In between the procedures, the wounds were covered with a four-layered wound dressing. 0.05 ml hydrogel (Nugel, Systagenix, North Yorkshire, England) were applied directly onto the wound in order to maintain a moist wound bed environment and the wound covered with Suprasorb F transparent moving-picture show dressing (Lohmann&Rauscher, Germany) (Fig. 1A). This functional bandage was stock-still with a two-layered retention cast (Hypafix and Leucoplast; Fig. 1B, C). Wound dressings were changed on day one, 3 and 6 post-surgery or whenever they became loose. Dressings were removed on day seven. Wound healing was tracked at least until day 7 and/or up to the day of wound closure.
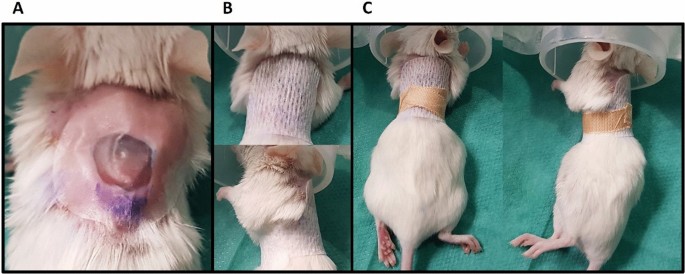
Functional 4-layered wound dressing to protect from external influences and continue a moist surroundings consisting of (A) Nugel hydrogel covered with Suprasorb F transparent picture dressing, which is fixed with (B) hypafix adhesive bandage and (C) leucoplast record.
Wound closure analysis
To follow the course of wound closure, digital 3D photos were taken at every wound dressing modify using a stereoscopic photographic camera (LifeViz micro, Quantificare, France). Wound area was analyzed by a planimetric measurement with a complimentary-paw tool using Republic of the fiji islands paradigm processing software (ImageJ, National Institute of Health, United states). The percent of the wound area was calculated using the post-obit formula:
$$Wound area \left[\%\right]= \left[\frac{wound expanse (day n)}{wound area (24-hour interval 0)} \correct]\times 100$$
where the wound area (day 0) is the surface area measured straight after surgery and wound expanse (day n) indicates the surface area on north days after surgery.
Quantitative microbiological analysis of CS
Bacteria in the cecal slurry were quantified past flow cytometry using the Leaner Counting Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Briefly, i vial of CS was centrifuged, and the pellet was resuspended in 500 µl 0.9% saline (1:16 dilution of the stock solution). The sample was further diluted 1:500 with 0.9% saline (1:8,000 of the stock solution) and 1 ml of this dilution was and then stained with 1 µl SYTO BC dye. For quality check, 10 µl of microsphere standard beads (half-dozen µm) were added to a second sample of the same dilution. After v min incubation at room temperature in the nighttime, the samples were analyzed using the flow cytometer (CytoFLEX AS16153, Beckman Coulter, CA, USA). The dye was excited using the laser emitting at 488 nm and the emission was recorded in the fluorescein channel.
Metagenome assay of CS via nanopore sequencing technology
The methodology and data analysis was recently described in a paper of Pinar et al.32.
Dna extraction
Total Deoxyribonucleic acid was isolated from 2 ml of CS. The slurry was vortexed for sample homogenization and then centrifuged (9,279 g, ten min, room temperature, Centrifuge 5415R, Eppendorf, Hamburg, Germany). The pellet (~ 100 mg) was subjected to DNA extraction using the FastDNA Spin Kit for Soil (MP Biomedicals, Illkirch-Graffenstaden, French republic) according to manufacturers' recommendations. To obtain the DNA purity and lucifer the Nanopore workflow's requirement, the DNA extract was further purified using the QIAamp Viral RNA Mini Kit (Qiagen, Venlo, Nederland). The DNA concentration was assessed past using the Qubit 2.0 Fluorometer with the Qubit dsDNA BR Assay Kit (Invitrogen, Carlsbad, The states).
(a) Library construction, template preparation and sequencing
Dna libraries were synthetic following the "1D Genomic DNA by Ligation" (SQK-LSK109) available in the Oxford Nanopore community using the Flow Jail cell Priming Kit EXP-FLP001 (Oxford Nanopore Technologies, Oxford, United Kingdom). All steps for library training were performed following the specifications of the protocol using ane µg input of extracted DNA. Following the preparation of the Dna ends for adapter zipper using the NEBNext FFPE Deoxyribonucleic acid Repair Mix (M6630, New England Biolabs, MA, United states) and the NEBNext End Repair/dA-Tailing Module (E7546 New England Biolabs, MA, Us), the attachment of sequencing adapters (supplied in the kit) to the Dna ends was accomplished by means of the NEBNext Quick Ligation Module (E6056, New England Biolabs, MA, USA).
After library preparation was completed, a flow cell quality control (FLO-MIN 106 R9 version, Oxford Nanopore Technologies, Oxford, United kingdom of great britain and northern ireland) was run prior to starting sequencing. The MinKNOW software (Oxford Nanopore Technologies, Oxford, United Kingdom) was used to cheque the number of agile pores in the flow jail cell. Finally, the priming and the loading of the DNA library into the menstruum jail cell were performed according to the recommendations of the manual supplied past the manufacturers.
The sequencing run was set as follows: the Nanopore device (MinION) was connected to a portable computer and the software MinKNOW was launched subsequently entering information about the sample (i.e. Sample and Catamenia cell ID) and selecting the advisable protocol script. Run was performed for 24 h.
(b) Data analyses
Resulting fast5 data files were basecalled using the Nanopore GPU basecalling with GUPPY three.0.3 on UBUNTU 16.04 (Oxford Nanopore Technologies Customs Platform, Oxford, United kingdom of great britain and northern ireland). In one case the Fastq files were generated, the data was compared with databases using i of the available pipelines for data analyses of the Nanopore Customs Platform, following the steps recommended by the manufacturers. The selected workflow chosen was "What's in my pot" (WIMP), which is an EPI2ME workflow for taxonomic classification of basecalled sequences (reads) generated by Nanopore sequencing. WIMP initially filters FASTQ files with a hateful q-score below a minimum threshold (defaults to 7). For reads above the quality threshold, the Centrifuge Classification Engine is executed to assign each read to a taxon in the NCBI taxonomy.
Microbiological swab analysis of the wound bed
A semi-quantitative microbiological swab analysis was conducted to narrate the wound infection. On twenty-four hour period 3 post-surgery, a sterile cotton swab (Sterile R, Meus S.R.L., Piove di Sacco, Italy) was used to take a representative microbiological sample from the wound area directly afterward the wound dressing removal. The samples were analyzed and adjudged by a veterinary diagnostics laboratory (Invitro, Vienna). This analysis included a total cultural differentiation and isolation with a post-obit identification via MALDI-TOF analysis. If necessary, a further biochemical differentiation according to the belittling profile index (API) was performed.
Wound score
For wound monitoring, a wound score was developed based on clinical observations and the wound closure. Table 1 shows the divers categories and the scoring criteria.
At each consecutive wound dressing change the wounds of all mice were evaluated and scored by two contained persons from 0 (best) to three (worst) according to Tabular array ane. The swelling of the wound edges, amount of liquid exudate, thickness of a yellow fibrin/pus layer, surface area covered, and thickness of formed scabs and severity of erythema were graded based on a visual inspection assessment. The sum of the wound scores was compared at dissimilar time points within the different experimental groups.
Autofluorescence imaging
Autofluorescence signals of the total-excision wounds were acquired daily (at dressing changes) using the loftier-performance multispectral Maestro in vivo imaging organization (CRI, Woburn, Massachusetts) at days 1–7 post-surgery. The autofluorescence signal of the CS colonies formed after incubation on LB agar petri dishes were used equally the reference bespeak to select the optimal procedure settings. Using the blue filter set (Thou-MSI-FLTR-Blueish, excitation 445–490 nm; emission 515 nm longpass), fluorescence was recorded from 500 to 720 nm in 10 nm steps for an automatically optimized exposure time (ms). The exposure time was kept constant for each mouse for all imaging time points. After linear unmixing of the autofluorescence point of interest (CS colony) from the one of fur and wound exudate within a defined and constant region of interest (ROI), the full signal in counts/s was analyzed and compared within the groups.
Statistical assay and data presentation
All statistical analyses were performed using the statistics software GraphPad (GraphPad Software Inc., Version v.0, CA, USA) and all data are presented as mean ± SD. P values of ≤ 0.05 were considered pregnant. The wound closure rates of wounds with and without bandages too as the wound scores among groups and fourth dimension points were compared using a two-way ANOVA of grouped analyses with Bonferroni correction to command for blazon I fault in multiple comparisons.
All figures and charts were prepared with Excel 2013 (Microsoft Corporation, WA, USA; https://www.microsoft.com) and GraphPad 5.0 (GraphPad Software Inc., CA, USA; https://www.graphpad.com).
Upstanding argument
All procedures were approved by the Animal Protocol Review Board of the Metropolis Authorities of Vienna, Austria (vote: 308358/2018/fifteen) and were in accordance with the National Institute of Health guidelines for the employ and intendance of laboratory animals. To ensure a comprehensive ascertainment, all animals were checked past trained professionals (i.e. DVMs and/or trained personnel) at least three times per solar day once they entered the experiment. Throughout the experiment, all mice received daily analgesic treatment (Meloxicam, i mg/kg, sid, s.c. or p.o.). In example of sustained signs of pain, mice were treated with buprenorphine (Bupaq, 0.1 mg/kg tid, s.c, Richter Pharma, Austria). All surgical procedures were done under inhalation anesthesia of iii–6% Sevoflurane (Sevorane, AbbVie Inc., Northward Chicago, Illinois, U.s.). Signs of systemic infection persisting for more than 24 h led to euthanasia of the fauna based on predefined humane endpoints. At the finish of the regular ascertainment flow all mice were killed under deep inhalation anesthesia with sevoflurane followed past cervical dislocation.
Results
Influence of the wound dressing on the wound
Since long-term experiments with mice are very challenging regarding the durability of wound dressings, we tested the necessity to comprehend wounds. All wounds healed inside 17 days irrespective of dressing.Although uncovered wounds seemed to close faster, which was even pregnant on day iii, the dry wound bed in this grouping was strongly associated with scab germination (Fig. 2B) and large wound expanse variations. In contrast, wounds covered with dressing showed a more homogenous wound healing process reflected by a continuously lower variation in wound size, i.east. at least twofold lower variation coefficient in dressed versus uncovered wounds (Fig. 2A). In accord with the clinical standard procedure for treatment of acutely infected wounds, all subsequent experiments were performed with wound dressing.
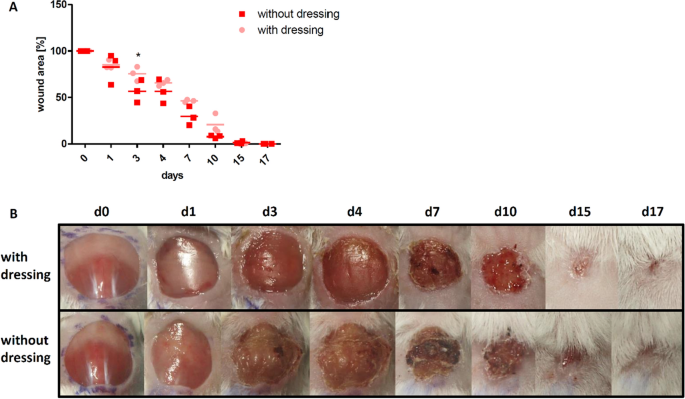
Influence of the wound dressing on the wound closure rate. (A) Wound closure charge per unit is slightly faster and less consequent in the group without dressing, showing a meaning departure on day iii. (B) Exemplary course of wound healing upwards to day 17 for a mouse with wound dressing and a mouse without wound dressing, showing the strong scab formation in the group without wound dressing. Mean ± SD; * P ≤ 0.05.
Influence of cyclophosphamide on wound infection and wound closure
Cyclophosphamide is a chemotherapeutic agent that induces neutropenia. To produce an environment of an inadequate microbial clearance (i.e. past reducing the neutrophil infiltration) with delayed wound healing, two-time intraperitoneal injection of a total dose of 250 mg/kg CPA was required earlier surgery/infection (Fig. 3A–E). We investigated a reduced CPA dosing to minimize systemic side effects, while maintaining the healing delay. All the same, without pretreatment or with lower doses of CPA, i.east. 83 mg/kg or 125 mg/kg, the allowed system remained active and bacterial infection was prevented, and so that wound healing speed was comparable to control mice without CPA (Fig. 3A–E).
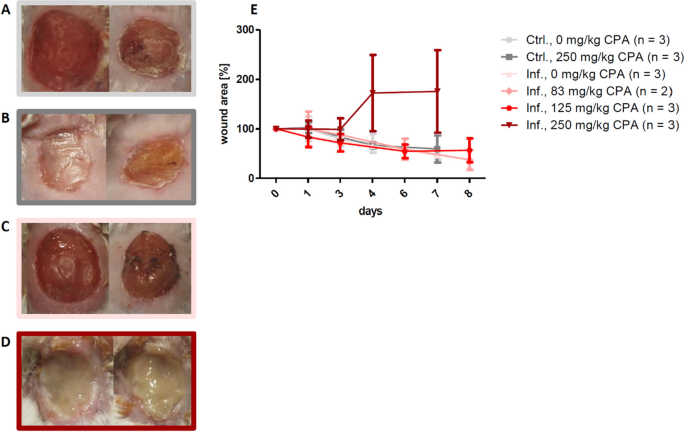
Influence of cyclophosphamide (CPA) induced neutropenia on the formation of wound infections. Exemplary wound pictures on day 5 (left) and twenty-four hour period 8 (right) in (A) the command group without CPA, (B) the control group with 250 mg/kg total dose of CPA, (C) the infection group without CPA and (D) the infection group with 250 mg/kg total dose of CPA. (E) 250 mg/kg CPA was needed to induce delayed wound healing. No pretreatment or reduction of the CPA dose to 83 mg/kg or 125 mg/kg total dose did prevent infection establishment and the wound healing charge per unit was like to the command group. Mean ± SD.
Influence of CS dose on wound infection and wound closure
After verifying the right CPA dose, several dilutions of CS inoculum were tested to find an optimal CS concentration ensuring a stable wound infection. Outset pilot studies showed that an inoculum of 30 µl of the undiluted CS stock solution (1 × ten9/ml leaner) or 1:sixteen diluted stock solution (6.25 × xvii/ml bacteria) in mice pretreated with 250 mg/kg CPA resulted in unwanted clinical signs of systemic infection. Based on the predefined humane endpoint criteria these mice were excluded from the experiment. In another setup the dilutions i:166, ane:400, 1:800 and 1:viii,000 of the CS stock were tested (Fig. 4). The 1:166 and 1:400 dilutions (between 6 × ten6/ml and 2.5 × ten6/ml leaner) contained the highest possible concentration of pathogens that resulted in comparable, delayed wound closure rates and well-established infections without whatsoever signs of systemic effects up to six days afterward infection.
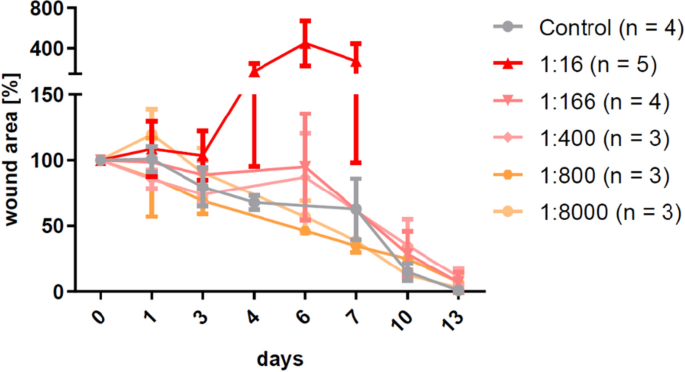
Influence of the cecal slurry (CS) concentration on the establishment of wound infections and the filibuster of wound healing. Strong systemic reaction rendered the stock concentration (1 × x9/ml bacteria) as well as the ane:xvi dilution unsuitable for the model and the i:800 and ane:8,000 dilutions were not showing whatsoever departure compared to the control group. Concentrations between ane:166 and one:400 (6 × 10vi to 2.5 × x6/ml leaner) contained the highest possible concentration of pathogens that showed a wound healing delay without causing signs of systemic reactions. Hateful ± SD.
Assessment of the established infection
Wound score, wound area, and swab analysis
Scoring was performed based on 3D photos in the control and the infection groups infected with CS dilutions of 1:166–1:400. For each brute, each single parameter was scored and the sum of all scores (for each individual mouse) was composed into a heat map (Fig. 5A). Wound healing score of infected mice was consistently elevated past at least 20% over the entire observation period (days 1–13) compared to controls. The difference was statistically significant on twenty-four hours six and 10 (p ≤ 0.05; Fig. 5B).
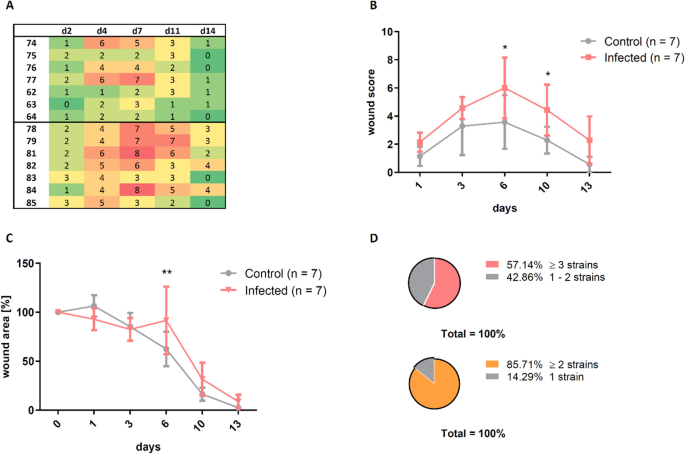
The wound score enabled the differentiation between the control grouping and the infected group. (A) The sum of the assigned scores for all parameters is presented every bit a heat map, where green shows a good and carmine a bad wound condition. (B) The continuous elevation of the score in the infection group was meaning on solar day half dozen and day ten. (C) The wound size in the infected mice was significantly increased on day six compared to the control group and was still trendwise enlarged on day x and day 13. (D) At to the lowest degree 2 different bacterial strains were determined in 86% of all wound infections and 57% of all infected wounds even showed at least 3 different strains of bacteria. Mean ± SD; P values: ** P ≤ 0.01, *P ≤ 0.05.
The bear on of infection on wound closure was confirmed by analysis of wound area, which was similarly increased in the infected group, showing a pregnant difference on twenty-four hours half dozen and trendwise increased wound areas on day 10 and 13 (Fig. 5C).
Analysis of microbiological swabs (summarized for all CPA mice) provided a characterization of the bacterial wound phenotype (Fig. 5D). In 86% of the wounds at least two bacterial species were detected, in 57% at least three species.
Effigy half dozen shows the comparison of bacterial species in the cecal slurry (Fig. 6A) and in the wounds (Fig. 6B) on 24-hour interval iii postal service-surgery. Notable is the occurrence of Staphylococcus species in some wounds, which were not detected in the CS. These ubiquitous species also established in wounds of control animals despite standard wound disinfection.
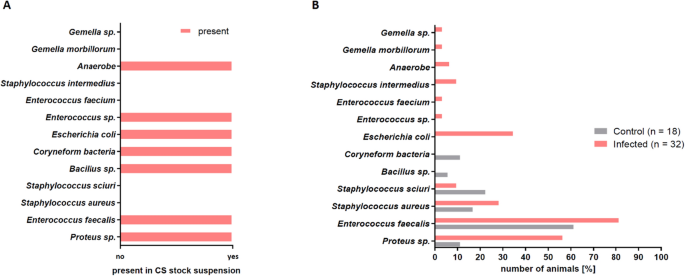
Comparison of the presence of different bacterial strains in the CS and the infected wounds. (A) Bacterial species detected in the CS analyzed before the infection procedure by microbiological swab analysis. (B) Bacterial species detected by microbiological swab analysis in the wound beds of control mice and wound beds of infected mice showed unlike profiles. Infected wounds showed a similar bacterial profile as the CS.
Additionally, in order to provide detailed information and label of the content of the contaminating suspension, a metagenome analysis based on 3rd generation sequencing of CS was performed (Supplementary Fig. 1). A comparison of the phylogenetic tree with a catalog of the mouse gut genome33 showed a good accordance with the genera contained in our CS suspension (Supplementary Fig. 1A). The metagenome showed that leaner constituted the largest fraction of well-nigh 55% of all reads assigned. Only a small fraction of 3.5% of bacterial genera of the CS were found in the wound beds analyzed past microbiological swab analysis, while among the others the anaerobic bacteria constituted a large fraction (Supplementary Fig. 1A). Still, this method was not suited to be routinely used in this study.
Autofluorescence imaging of wound infections
Recently, autofluorescence imaging was introduced to detect bacterial wound infections34. A secondary aim of this study was to exam an in vivo fluorescence Maestro imaging arrangement (CRi Inc., Woburn, Mass, USA) for the convenient tracking and quantification of the wound infections. First in vitro tests using CS smears on LB agar plates confirmed strong autofluorescence point of all colonies visible on the plates (Fig. 7A).
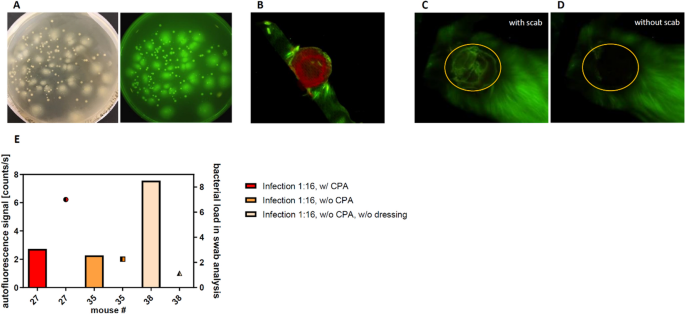
Autofluorescence tracking of wound infections. (A) Cecal slurry colonies showed a strong fluorescence signal in vitro. (B) By linear unmixing the wound exudate (scarlet) and the autofluorescence signal of the fur (green) could be differentiated from the autofluorescence of the infection. (C, D) The scab formation strongly influenced the quantification of the infection autofluorescence. (Due east) The accord of the quantified autofluorescence (bars) with the results of the microbiological wound swabs (dots) on day 3 post surgery was poor.
However, in vivo data showed that the fluorescence signal was not specific plenty for the detection of bacterial infection. The wound exudate, which was mainly observed on days i–ii mail-surgery, gave a stiff positive point, for case on the wound dressings shown in Fig. 7B. A longer follow-upwardly fourth dimension series over seven days revealed that also scab germination significantly hampers the autofluorescence signal (Fig. 7C, D).
Accordingly, on day iii post-surgery the autofluorescence signal (Fig. 7E, confined) of the wound did not correspond to the semi-quantitative results of the microbiological swab analysis (Figs. 7E, dots). For example, the wounds with the highest autofluorescence signal (Fig. 7E, lite yellow bar) showed a low quantity in the microbiological swab analysis (Fig. 7E, lite yellow dot).
Word
In the setting of a compromised allowed arrangement, an exposure of fresh wounds to microbes may lead to severe infections and delayed wound healing35. Well-nigh of the currently existing wound models dealing with bacterial infections used either single pathogen species or combinations of selected bacterial strains29,36,37,38. However, under clinical atmospheric condition, patients are often confronted with complex wound infections, even though one strain might ultimately predominate. Wolcott et al. institute that in a cohort of near 3,000 chronic wound patients, 93% of the wound microbiomes were polymicrobial39. The authors investigated infections of ulcer wounds and detected a loftier proportion of Staphylococcus and Pseudomonas species in 63% and 25% of all wounds, respectively, but likewise observed a high prevalence of anaerobic bacteria and bacteria traditionally considered commensalistic. Similarly, Kalan and Brennanforty reviewed the role of the microbiome in nonhealing diabetic wounds and therein mentioned a written report from Citron et al., which analyzed 454 diabetic foot ulcers and found a fraction of over 80% to be polymicrobial41. However, preclinical models simulating infections induced by a naturally occurring polymicrobial inoculum are missing. Therefore, this written report aimed to establish a wound infection model in mice that includes the factor of naturally occurring microbial customs interactions. For contagion a previously prepared and cryopreserved cecal slurry from carrion of the same species was used. The pick of this methods was supported past a publication of Tipton et al. showing no significant influence in community composition afterward cryogenic preservation, whereas a significant loss of diversity was plant when wound communities were transferred between species from human to mouse42. The complication of this model provides the opportunity to afterwards test advisable therapeutic approaches.
Various forms of immunodeficiency (existing as a built and/or acquired comorbidity) may institute a serious impediment to the wound healing process given their poor tissue regenerative potential and/or low capacity for microbial clearance. For instance, recipients of organ transplants43, and individuals suffering from human immunodeficiency virus/acquired immunodeficiency syndrome44,45 typically display an dumb wound healing46. A mutual feature of the native immune deficit includes an impaired infiltration/migration of granulocytes (predominantly neutrophils) to the infection site to clear the invading microorganisms47. Cyclophosphamide is a chemotherapeutic drug that is in clinical use for handling of cancer and autoimmune diseases. Its administration induces neutropenia and lymphopenia, and therefore in the present written report facilitated bacterial wound infections in mice by inhibiting the innate immune response31,48,49. Similar to other enquiry groups, who studied unmarried-strain infections31,49, we showed that immunosuppression with CPA is a necessary prerequisite for the institution of severe polymicrobial wound infections using the CS inoculum. In contrast to some studies indicating a delayed wound healing after this pretreatment23,50,51, in the present study CPA by itself had no issue on the wound closure rate.
In pilot experiments, the infection with high CS doses (undiluted stock CS containing i × 10nine/ml bacteria) in CPA-immunosuppressed mice resulted in clinical symptoms of severe systemic infection. These findings are in line with Thompson et al., who too faced septic events in a mouse model of wound infection with high doses of A. baumanni 52. Our results showed that in accordance with Zululaga et al.31, who tested the same CPA dose in outbred ICR mice, the optimal CPA dose for reliable leukopenia in BALB/c mice was 250 mg/kg. Lower doses of CPA did not result in persistent signs of bacterial infection of the wound based on the wound healing score. However, CPA doses should exist optimized for each individual infection model, animal species and strain to avoid systemic side effects. The dependence of infection-delayed wound closure on the CPA dose seemed to resemble rather an on–off-machinery than a gradually configurable system. Therefore, fine-tuning of infection severity was performed in a second pace by the gradual lowering of the CS inoculation dose. A like process was previously reported in an allowed-suppressed murine model infected with Acinetobacter baumannii, where sublethal doses (based on a normal allowed organization status) of leaner had to be reduced from 107 CFU to 3.75 × 106 CFU to lower the mortality rate from 100 to 20%49.
Besides the intended infection with known enterobacterial strains from the CS, some wound swabs showed boosted contamination/infection with some ubiquitous microorganisms (i.e. predominantly South. aureus). This was problematic equally S. aureus infections competed with bacterial strains from the CS inoculum and were able to overgrow them occasionally. The cooperative and competitive interactions of Due south. aureus with other bacterial strains and its altered behavior and increased persistency in polymicrobial communities were comprehensively reviewed by Nair et al.53 Thus, in the present written report mice with infections solely showing ubiquitous bacterial strains that were not part of the CS inoculum based on the microbiological swab analysis were excluded.
In order to avoid auto-infections of the wounds with skin-resident bacterial flora, to achieve maximum difference between infected and non-infected wounds and to ensure optimal reproducible conditions, nosotros decided to administer topical wound disinfection with Octenidindihydrochlorid (Octenisept). This recapitulates the electric current clinical do given that wound disinfection has become a standard aim to prevent and manage inpatient wound infections as well as to reduce antibiotics and forestall development of antimicrobial resistance54,55. The presence/absence of the topical disinfection in control (uninfected) animals did not influence the wound closure speed (data not shown).
For basic enquiry on bacterial infections, the utilise of luminescent bacterial strains and the detection by bioluminescence imaging are widely used tools22,56,57. Still, as this procedure requires transiently transformed bacteria and in the present study nosotros aimed to apply a complex biological sample (CS) for contagion, bioluminescence imaging was no option. Withal, tracking bioluminescence signals of transiently transformed leaner in long-term in vivo studies is rather impossible due to two main reasons: (a) an comparable transformation with similar bioluminescent signal cannot be reached for all bacterial species in biological samples, like CS, (b) stable expression and a reliable quantitative signal could not exist guaranteed since the selective pressure cannot exist maintained over several days. Therefore, we aimed to establish a multifactorial analysis tool to assess the touch on of feces-induced infections on wound healing including microbiological swab assay, wound closure measurement, a newly established wound score and also tested autofluorescence imaging.
Real-time monitoring of wound infections past autofluorescence imaging is a tool for clinical diagnostics. Both preclinical34 and clinical58,59 studies showed that autofluorescence-guided sampling confirmed the proper selection of antibacterial treatment strategies. In the present study a similar arroyo was tested using in vivo autofluorescence imaging for the follow-up detection of feces-induced infections. The Maestro imaging device allows to analyze autofluorescence in anaesthetized rodents. In vitro, the betoken of the cultivated CS-derived colonies was strong and adequately defined. All the same, in vivo the autofluorescence signal strength did non correspond with the results of the standard microbiological cultures. The authors assume that the bacterial load in the wound was not high enough for reliable detection given that devices for clinical employ are reported to exist best for the detection of moderate to heavy bacterial growth59. Since the early assessment of infections is crucial for preventive handling approaches, we refrained from further utilise of autofluorescence imaging in this model setup. Thus, we supplemented the quantitative assay of wound closure and microbiological swab analysis with a qualitative analysis using a wound healing score. We modified previously published wound scores60,61 to depict the differences observed between control and infected wounds past assigning them to five different categories with a four-point scale. The summarized score was continuously significantly increased in the infection group. Eventually the comprehensive wound evaluation, including wound closure analysis, microbiological swab analysis and the wound healing score, enabled a reliable differentiation betwixt control and infection group.
In determination, in this study nosotros nowadays the establishment of a uncomplicated and reliable in vivo skin wound model, infected with a naturally occurring bacterial suspension. The combination of quantitative and qualitative wound analyses guarantees a comprehensive evaluation of the infection and wound healing progress. Therefore, this model can be used for the testing of infection-control therapies every bit well as strategies to improve infection-dumb wound healing and thereby bulldoze the supporting and/or alternative treatment approaches to gear up for the postal service-antibiotic era.
References
-
Report Global Wound Dressings Market 2018–2022, TechNavio, Infiniti Research Ltd., London, UK (2018)
-
Gottrup, F., Apelqvist, J., Cost, P. & European Wound Direction Clan Patient Issue, M. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to better the quality of testify in wound management. J Wound Care nineteen, 237–268. https://doi.org/x.12968/jowc.2010.xix.6.48471 (2010).
-
Sunderkotter, C. & Becker, 1000. Frequent bacterial skin and soft tissue infections: Diagnostic signs and treatment. J Dtsch Dermatol Ges 13, 501–524. https://doi.org/10.1111/ddg.12721 (2015) ((quiz 525–506)).
-
Arrangement, Due west. H. WHO | WHO Global Strategy for Containment of Antimicrobial Resistance. WHO (2016).
-
Neil, J. A. Perioperative care of the immunocompromised patient. AORN J 85, 544–560. https://doi.org/10.1016/S0001-2092(07)60126-four (2007) ((quiz 561–544)).
-
Livesley, North. J. & Chow, A. W. Infected pressure ulcers in elderly individuals. Clin. Infect. Dis. 35, 1390–1396. https://doi.org/10.1086/344059 (2002).
-
Pendleton, J. N., Gorman, S. P. & Gilmore, B. F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 11, 297–308. https://doi.org/ten.1586/eri.13.12 (2013).
-
Santajit, S. & Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016, 2475067. https://doi.org/10.1155/2016/2475067 (2016).
-
Dai, T. et al. Creature models of external traumatic wound infections. Virulence 2, 296–315. https://doi.org/10.4161/viru.2.four.16840 (2011).
-
Tatara, A. Grand., Shah, S. R., Livingston, C. E. & Mikos, A. G. Infected animate being models for tissue engineering. Methods 84, 17–24. https://doi.org/10.1016/j.ymeth.2015.03.025 (2015).
-
Dai, T., Tegos, G. P., Zhiyentayev, T., Mylonakis, E. & Hamblin, Thou. R. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse peel abrasion model. Lasers Surg. Med. 42, 38–44. https://doi.org/10.1002/lsm.20887 (2010).
-
Zolfaghari, P. S. et al. In vivo killing of Staphylococcus aureus using a low-cal-activated antimicrobial agent. BMC Microbiol. 9, 27. https://doi.org/10.1186/1471-2180-9-27 (2009).
-
Kraft, Westward. G., Johnson, P. T., David, B. C. & Morgan, D. R. Cutaneous infection in normal and immunocompromised mice. Infect. Immun. 52, 707–713 (1986).
-
Kugelberg, E. et al. Establishment of a superficial peel infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 49, 3435–3441. https://doi.org/10.1128/AAC.49.8.3435-3441.2005 (2005).
-
Gaspari, A. A. et al. CD86 (B7–2), merely not CD80 (B7–one), expression in the epidermis of transgenic mice enhances the immunogenicity of primary cutaneous Candida albicans infections. Infect. Immun. 66, 4440–4449 (1998).
-
Jeray, K. J. et al. Evaluation of standard surgical preparation performed on superficial dermal abrasions. J. Orthop. Trauma fourteen, 206–211 (2000).
-
Walker, H. Fifty. & Mason, A. D. Jr. A standard animal fire. J. Trauma 8, 1049–1051. https://doi.org/10.1097/00005373-196811000-00006 (1968).
-
Stieritz, D. D. & Holder, I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: Description of a burned mouse model. J. Infect. Dis. 131, 688–691. https://doi.org/ten.1093/infdis/131.6.688 (1975).
-
Katakura, T., Yoshida, T., Kobayashi, M., Herndon, D. N. & Suzuki, F. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin. Exp. Immunol. 142, 419–425. https://doi.org/10.1111/j.1365-2249.2005.02944.ten (2005).
-
Stevens, E. J. et al. A quantitative model of invasive Pseudomonas infection in burn injury. J. Burn Care Rehabil. 15, 232–235 (1994).
-
Manafi, A. et al. Active immunization using exotoxin A confers protection against Pseudomonas aeruginosa infection in a mouse burn model. BMC Microbiol. 9, 23. https://doi.org/x.1186/1471-2180-9-23 (2009).
-
Hamblin, Chiliad. R., O'Donnell, D. A., Murthy, N., Contag, C. H. & Hasan, T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem. Photobiol. 75, 51–57 (2002).
-
Burkatovskaya, M., Castano, A. P., Demidova-Rice, T. North., Tegos, G. P. & Hamblin, M. R. Consequence of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. sixteen, 425–431. https://doi.org/x.1111/j.1524-475X.2008.00382.x (2008).
-
Simonetti, O. et al. RNAIII-inhibiting peptide enhances healing of wounds infected with methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 52, 2205–2211. https://doi.org/10.1128/AAC.01340-07 (2008).
-
Mahoney, E. et al. Bacterial colonization and the expression of inducible nitric oxide synthase in murine wounds. Am. J. Pathol. 161, 2143–2152. https://doi.org/x.1016/s0002-9440(x)64492-6 (2002).
-
Bowler, P. G., Duerden, B. I. & Armstrong, D. Thou. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 14, 244–269. https://doi.org/10.1128/CMR.fourteen.2.244-269.2001 (2001).
-
Klein, P. et al. A porcine model of skin wound infected with a polybacterial biofilm. Biofouling 34, 226–236. https://doi.org/x.1080/08927014.2018.1425684 (2018).
-
Mastropaolo, Chiliad. D. et al. Synergy in polymicrobial infections in a mouse model of type 2 diabetes. Infect. Immun. 73, 6055–6063. https://doi.org/10.1128/IAI.73.ix.6055-6063.2005 (2005).
-
Dalton, T. et al. An in vivo polymicrobial biofilm wound infection model to written report interspecies interactions. PLoS ONE six, e27317. https://doi.org/10.1371/journal.pone.0027317 (2011).
-
Starr, M. E. et al. A new cecal slurry training protocol with improved long-term reproducibility for creature models of sepsis. PLoS ONE 9, e115705. https://doi.org/ten.1371/journal.pone.0115705 (2014).
-
Zuluaga, A. F. et al. Neutropenia induced in outbred mice past a simplified depression-dose cyclophosphamide regimen: Characterization and applicability to diverse experimental models of infectious diseases. BMC Infect. Dis. 6, 55. https://doi.org/10.1186/1471-2334-6-55 (2006).
-
Piñar, M., Poyntner, C., Lopandic, Grand., Tafer, H. & Sterflinger, K. Rapid diagnosis of biological colonization in cultural artefacts using the MinION nanopore sequencing technology. Int. Biodeterior. Biodegrad. 148, 104908. https://doi.org/10.1016/j.ibiod.2020.104908 (2020).
-
Xiao, Fifty. et al. A itemize of the mouse gut metagenome. Nat Biotechnol 33, 1103–1108. https://doi.org/x.1038/nbt.3353 (2015).
-
Wu, Y. C. et al. Autofluorescence imaging device for real-time detection and tracking of pathogenic bacteria in a mouse pare wound model: preclinical feasibility studies. J. Biomed. Opt. nineteen, 085002. https://doi.org/ten.1117/one.JBO.19.8.085002 (2014).
-
Kline, G. A. & Bowdish, D. M. Infection in an aging population. Curr. Opin. Microbiol. 29, 63–67. https://doi.org/10.1016/j.mib.2015.11.003 (2016).
-
Hamblin, M. R. et al. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 49, 941–951. https://doi.org/10.1093/jac/dkf053 (2002).
-
Hamblin, M. R., Zahra, T., Contag, C. H., McManus, A. T. & Hasan, T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J. Infect. Dis. 187, 1717–1725. https://doi.org/ten.1086/375244 (2003).
-
Dai, T. et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob. Agents Chemother. 53, 3929–3934. https://doi.org/10.1128/AAC.00027-09 (2009).
-
Wolcott, R. D. et al. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 24, 163–174. https://doi.org/10.1111/wrr.12370 (2016).
-
Kalan, Fifty. R. & Brennan, M. B. The part of the microbiome in nonhealing diabetic wounds. Ann. N. Y. Acad. Sci. 1435, 79–92. https://doi.org/10.1111/nyas.13926 (2019).
-
Citron, D. M., Goldstein, E. J., Merriam, C. V., Lipsky, B. A. & Abramson, One thousand. A. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J. Clin. Microbiol. 45, 2819–2828. https://doi.org/x.1128/JCM.00551-07 (2007).
-
Tipton, C. D. et al. Chronic wound microbiome colonization on mouse model following cryogenic preservation. PLoS Ane 14, e0221565. https://doi.org/10.1371/journal.pone.0221565 (2019).
-
Roine, E., Bjork, I. T. & Oyen, O. Targeting risk factors for impaired wound healing and wound complications after kidney transplantation. Transplant Proc. 42, 2542–2546. https://doi.org/10.1016/j.transproceed.2010.05.162 (2010).
-
Abalo, A. et al. Risk factors for surgical wound infection in HIV-positive patients undergoing surgery for orthopaedic trauma. J. Orthop. Surg. (Hong Kong) 18, 224–227. https://doi.org/10.1177/230949901001800218 (2010).
-
Davis, P. A., Corless, D. J., Gazzard, B. M. & Wastell, C. Increased take chances of wound complications and poor healing following laparotomy in HIV-seropositive and AIDS patients. Dig. Surg. sixteen, 60–67. https://doi.org/x.1159/000018695 (1999).
-
Guo, S. & Dipietro, Fifty. A. Factors affecting wound healing. J. Dent. Res. 89, 219–229. https://doi.org/10.1177/0022034509359125 (2010).
-
Su, Y. & Richmond, A. Chemokine regulation of neutrophil infiltration of pare wounds. Adv. Wound Care (New Rochelle) 4, 631–640. https://doi.org/10.1089/wound.2014.0559 (2015).
-
Gad, F., Zahra, T., Francis, K. P., Hasan, T. & Hamblin, Grand. R. Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem. Photobiol. Sci. three, 451–458. https://doi.org/10.1039/b311901g (2004).
-
Manepalli, Southward. et al. Characterization of a cyclophosphamide-induced murine model of immunosuppression to written report Acinetobacter baumannii pathogenesis. J. Med. Microbiol. 62, 1747–1754. https://doi.org/10.1099/jmm.0.060004-0 (2013).
-
Bairy, Fifty., Ganesh, S. B., Adiga, Due south. & Shalini, A. Impaired wound healing due to cyclophosphamide (CLP) alleviated by supplemental Ginkgo biloba (GB). J. Nat. Remed. 6, 31–34 (2006).
-
Wie, H., Bruaset, I. & Eckersberg, T. Effects of cyclophosphamide on open up, granulating peel wounds in rats. Acta Pathol. Microbiol. Scand. A 87A, 185–192 (1979).
-
Thompson, M. G. et al. Validation of a novel murine wound model of Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 58, 1332–1342. https://doi.org/x.1128/AAC.01944-13 (2014).
-
Nair, N., Biswas, R., Gotz, F. & Biswas, L. Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections. Infect. Immun. 82, 2162–2169. https://doi.org/10.1128/IAI.00059-xiv (2014).
-
Leise, B. S. Topical wound medications. Vet. Clin. N. Am. Equine Pract. 34, 485–498. https://doi.org/10.1016/j.cveq.2018.07.006 (2018).
-
Barrett, S. Wound-bed grooming: A vital pace in the healing process. Br. J. Nurs. 26, S24–S31. https://doi.org/ten.12968/bjon.2017.26.12.S24 (2017).
-
Garcez, A. Due south. et al. Effects of photodynamic therapy on Gram-positive and Gram-negative bacterial biofilms by bioluminescence imaging and scanning electron microscopic assay. Photomed Light amplification by stimulated emission of radiation Surg. 31, 519–525. https://doi.org/10.1089/pho.2012.3341 (2013).
-
Wang, Y. et al. In vivo investigation of antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii burn infections using bioluminescence imaging. J. Vis. Exp. https://doi.org/10.3791/54997 (2017).
-
DaCosta, R. Southward. et al. Point-of-care autofluorescence imaging for existent-time sampling and treatment guidance of bioburden in chronic wounds: First-in-human results. PLoS ONE ten, e0116623. https://doi.org/10.1371/periodical.pone.0116623 (2015).
-
Ottolino-Perry, K. et al. Improved detection of clinically relevant wound bacteria using autofluorescence image-guided sampling in diabetic foot ulcers. Int. Wound J fourteen, 833–841. https://doi.org/10.1111/iwj.12717 (2017).
-
Jamadagni, P. South. et al. Experimental and histopathological observation scoring methods for evaluation of wound healing backdrop of Jatyadi Ghrita. Ayu 37, 222–229. https://doi.org/10.4103/ayu.AYU_51_17 (2016).
-
Panuncialman, J. & Falanga, V. The science of wound bed preparation. Clin. Plast. Surg. 34, 621–632. https://doi.org/10.1016/j.cps.2007.07.003 (2007).
Acknowledgements
We thank Carina Wagner for valuable technical support.
Author information
Affiliations
Contributions
L.K., S.D. and P.D. conceived and designed the inquiry. L.Thou., Due south.D., M.M., J.Z. and P.D. performed the experiments and contributed to the manuscript. Fifty.K., M.M. and P.S. supported and performed data analyses. J.Grand. and Thousand.O. supported the written report by providing critical input and knowledge in the field disquisitional care and wound healing. 1000.South and G.P. performed Nanopore analyses and provided Fig. vi and Supplementary Figure ane. F.L. supported with statistical analysis Fifty.K., S.D. and P.D. wrote the manuscript with revisions from all authors. Southward.D. and P.D. supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Admission This article is licensed under a Creative Eatables Attribution iv.0 International License, which permits utilize, sharing, accommodation, distribution and reproduction in any medium or format, as long as yous give appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The images or other tertiary party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the textile. If material is not included in the article'southward Creative Commons license and your intended use is not permitted past statutory regulation or exceeds the permitted apply, y'all will need to obtain permission straight from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Well-nigh this commodity
Cite this article
Karner, L., Drechsler, Due south., Metzger, Chiliad. et al. Contamination of wounds with fecal bacteria in immuno-suppressed mice. Sci Rep 10, 11494 (2020). https://doi.org/10.1038/s41598-020-68323-5
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-020-68323-five
Comments
By submitting a comment you agree to bide past our Terms and Customs Guidelines. If yous detect something abusive or that does not comply with our terms or guidelines delight flag it equally inappropriate.
Source: https://www.nature.com/articles/s41598-020-68323-5
Posted by: martintagazier1947.blogspot.com


0 Response to "How To Clean Wound Contaminated With Feces"
Post a Comment